Efficacy and safety of Cibinqo
Cibinqo (abrocitinib) is a JAK1 inhibitor that works by blocking specific chemical signal pathways, thus reducing skin inflammation and providing symptom relief to patients.1–3
It has been extensively studied in various clinical trials, providing evidence that Cibinqo is effective as a monotherapy or in combination with topical therapies^ in adult patients.1,4–7
- ^Topical therapies include topical corticosteroids (medium or low potency), topical calcineurin inhibitors, or topical phosphodiesterase-4 (PDE4) inhibitors.6,7
Relief symptoms with Cibinqo
The benefits of Cibinqo can be consistently observed across several clinical trials.4–7
Efficacy of Cibinqo as a monotherapy
(compared with placebo)4,5,8#
After 3 months of Cibinqo,
about
5x
more people saw a 75% skin improvement in symptoms such as redness and swelling4,5

§p<0.0001; ¶p<0.001
Adapted from Simpson et al. 2020 & Silverberg et al. 2020.4,5
- *JADE MONO-1 is a phase 3 clinical trial with 387 patients aged ≥12 years from Australia, Canada, Europe, and USA.4
- †JADE MONO-2 is a phase 3 clinical trial with 391 patients aged ≥12 years from Australia, Bulgaria, Canada, China, Czechia, Germany, Hungary, Japan, South Korea, Latvia, Poland, United Kingdom, and USA.5
After 3 months of Cibinqo,
about
4x
more people achieved ‘clear’ or ‘almost clear’ skin (ie, relief of symptoms such as redness and swelling)4,5

§p<0.0001; ¶p<0.001
Adapted from Simpson et al. 2020 & Silverberg et al. 2020.4,5
- *JADE MONO-1 is a phase 3 clinical trial with 387 patients aged ≥12 years from Australia, Canada, Europe, and USA.4
- †JADE MONO-2 is a phase 3 clinical trial with 391 patients aged ≥12 years from Australia, Bulgaria, Canada, China, Czechia, Germany, Hungary, Japan, South Korea, Latvia, Poland, United Kingdom, and USA.5
Within 2 weeks,
about
40
%
of patients experienced itch relief with Cibinqo vs about 3% of those on placebo4,5
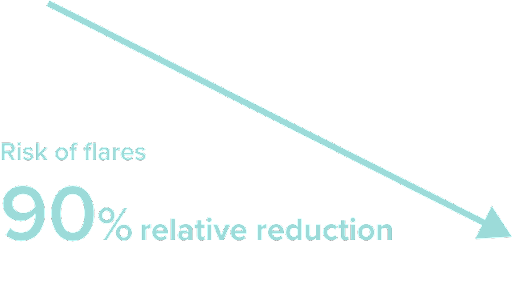
Continuously taking Cibinqo through Week 52 led to a
90
%
relative reduction
in the risk of flares vs placebo8
- #Note: Cibinqo is for people aged 18 years and above.1
Efficacy of Cibinqo as a combination with topical therapy^
(compared with a biologic + topical therapy)7,9
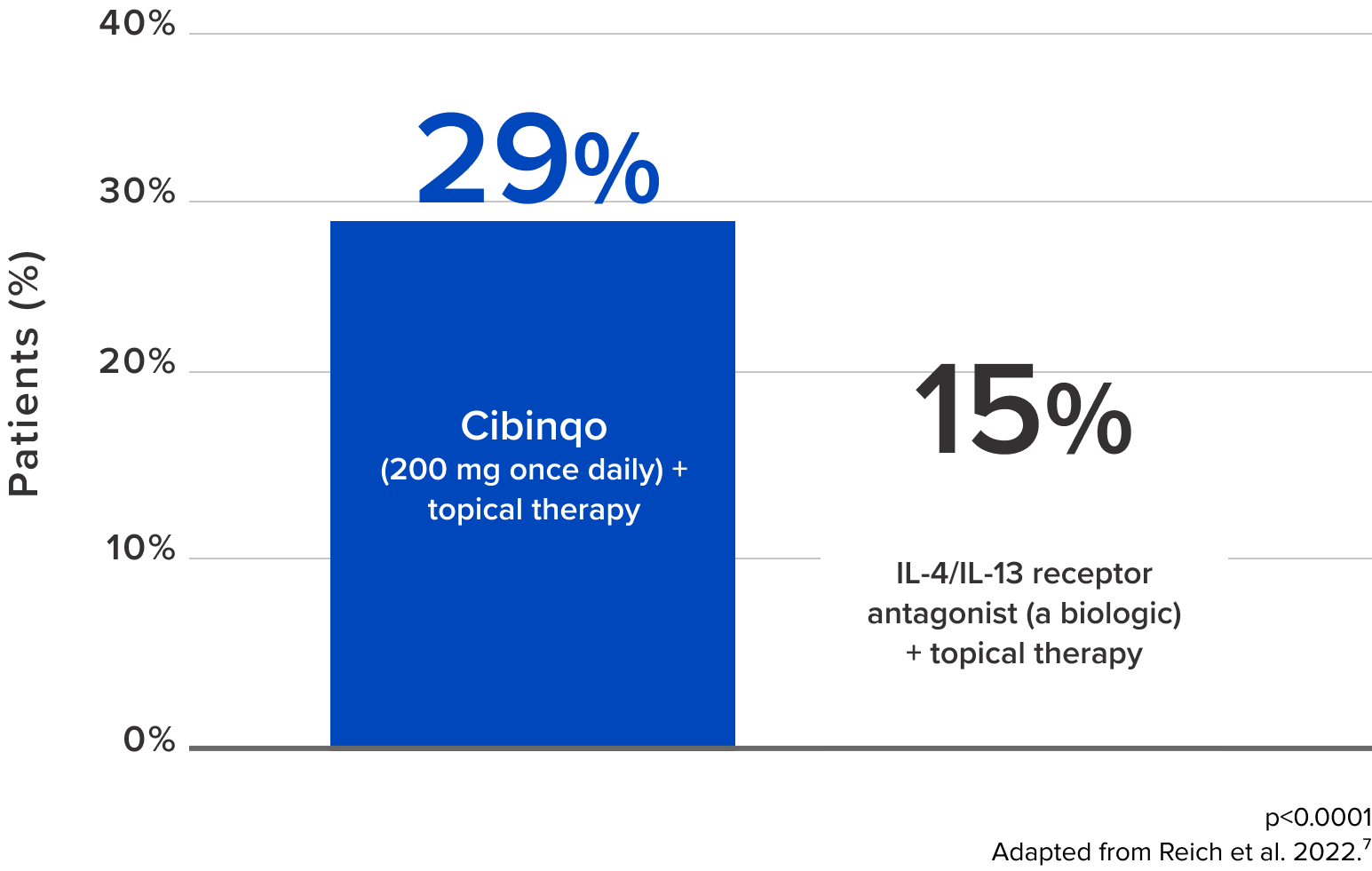
After 1 month of Cibinqo +
topical therapy,
about
2x
more patients saw a 90% skin improvement in symptoms such as redness and swelling7
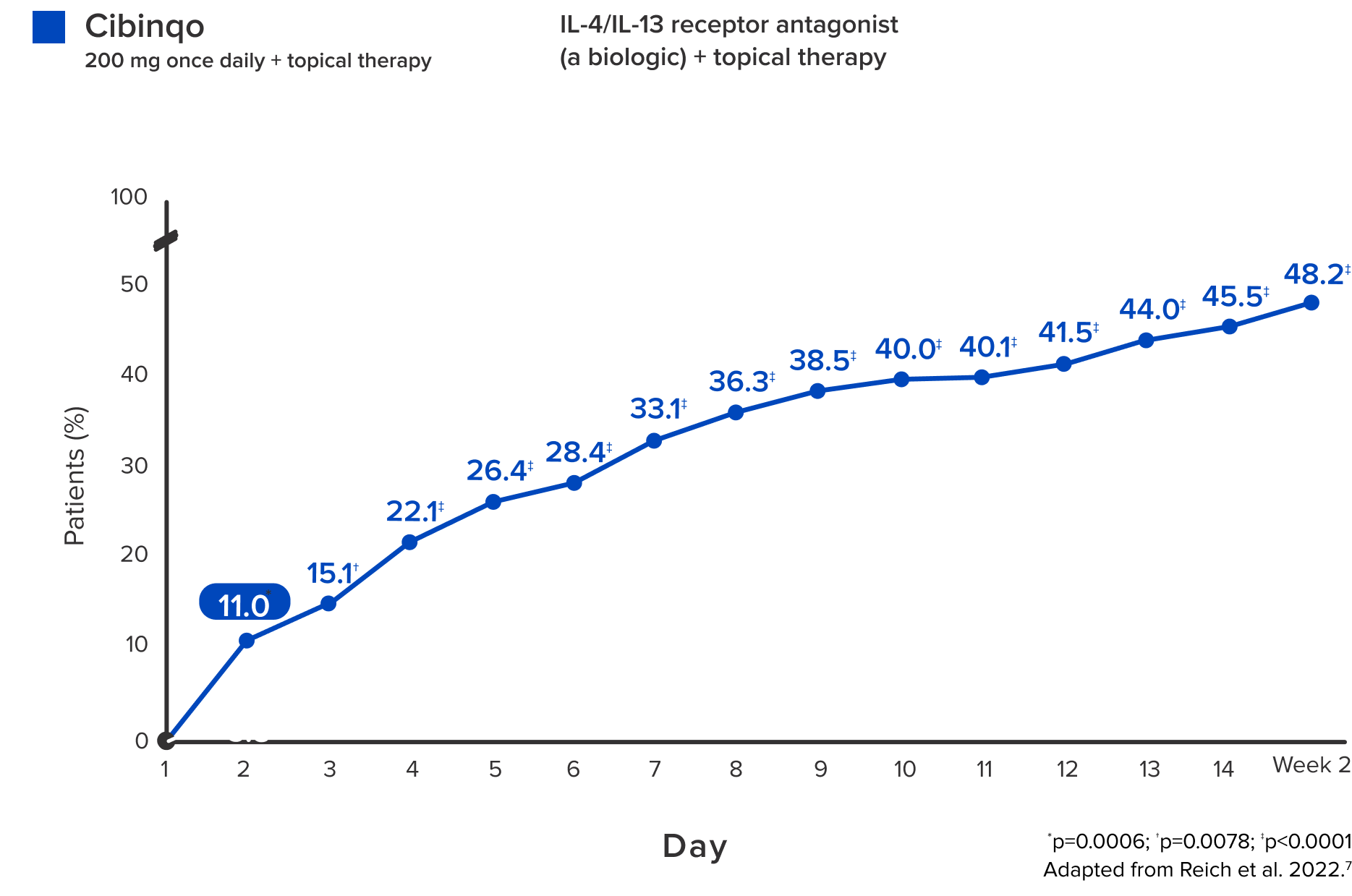
Significant itch relief was achieved
as fast as the
second day
with Cibinqo + topical therapy, with 2x as many patients in 2 weeks than patients on biologic + topical therapy7
Among patients who achieved response at 3 months10#:
-

87%- continued to achieved
skin clearance - (ie, relief of symptoms such as redness and swelling) after another year of Cibinqo
-

83%- continued to attained
itch relief - after another year of Cibinqo
- #Note: Cibinqo is for people aged 18 years and above.1
- ^Topical therapies include topical corticosteroids (medium or low potency), topical calcineurin inhibitors, or topical phosphodiesterase-4 inhibitors.6,7
Improve your life quality with Cibinqo
Across clinical trials, patients taking Cibinqo have reported significant improvement in their quality of life.1,6,11,12
An improvement in health-related quality of life after 12 weeks of Cibinqo was observed11,12

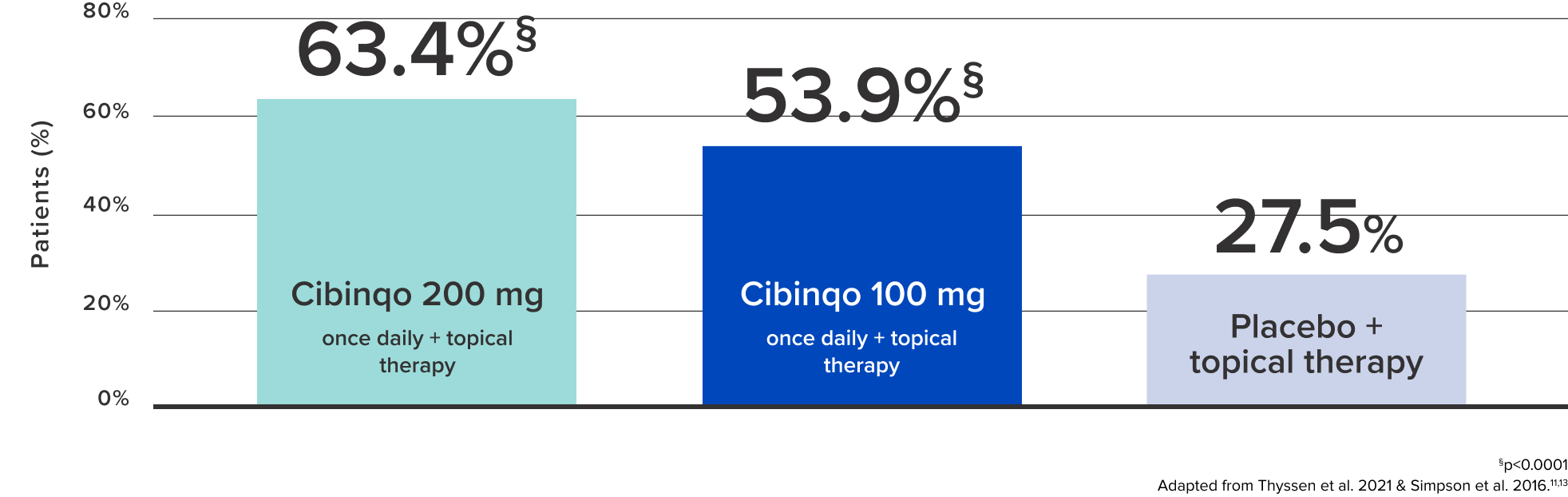
Compared with placebo, more than 2x of the patients reported a rapid improvement in sleep condition after 12 weeks of Cibinqo11,13

Side effects of Cibinqo
As with all other medications, Cibinqo can cause side effects.14 The most common ones include nausea, headache, acne, herpes simplex infection, increased blood creatine phosphokinase (an enzyme in the blood), vomiting, dizziness and upper abdominal pain.1
Cibinqo should be used according to its prescribing information.14 Discuss with your doctor if you have any concerns about drug-related side effects.
References
- CIBINQO® (abrocitinib) Prescribing Information. Pfizer Corporation Hong Kong Limited: Version September 2022.
- Hu X, et al. Signal Transduct Target Ther 2021;6:402.
- NEA. FAQ – Cibinqo (abrocitinib). Available at: nationaleczema.org/faq-cibinqo-abrocitinib/. Accessed Jan 2023.
- Simpson EL, et al. Lancet 2020;396:255-266. (JADE MONO-1)
- Silverberg JL, et al. JAMA Dermatol 2020;156:863-873. (JADE MONO-2)
- Bieber T, et al. N Engl J Med 2021;384:1101-1112. (JADE COMPARE)
- Reich K, et al. Lancet 2022;400:273-282. (JADE DARE)
- Blauvelt A, et al. J Am Acad Dermatol 2022;86:104-112. (JADE REGIMEN)
- Shi VY, et al. J Am Acad Dermatol 2022;87:351-358. (JADE EXTEND)
- Reich K, et al. J Eur Acad Dermatol Venereol 2023;37:2056-2066. (JADE EXTEND)
- Thyssen JP, et al. J Eur Acad Dermatol Venereol 2022;36:434-443.
- Basra MKA, et al. Dermatology 2015;230:27-33.
- Simpson EL, et al. J Am Acad Dermatol 2016;74:491-498.
- CIBINQO® (abrocitinib) Package Leaflet. Pfizer Corporation Hong Kong Limited: Version September 2022.